The opisthobranchs are simultaneous hermaphrodites. The genital atrium is, in general, in the front right part, which is the reason why the individuals more or less face each other for copulation and show this side.
Two specimens of Trapania hispalensiscopulating on the sponge Sarcotragus spinosulus. In the one on the left, a parasitic copepod is seen by transparency and between the gills its setting. (Accelerated sequence x3)
|
|
|

Sea hares are one of the exceptions to this form of copulation, having a channel that leads the sperm from the dorsal part, where the genital atrium is, to a kind of penis located in the right frontal part. The fact that the penis and vagina are separated allows for intercourse to happen in chain form.
|
|
|
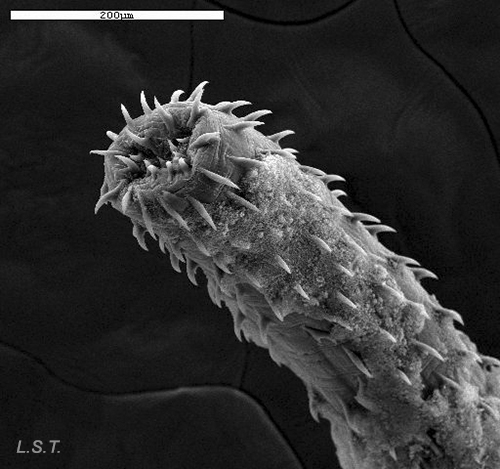
In some species, the penis may be armed with spines, like the one in the photograph belonging to a dorid-dove of the species Tyrannodoris europaea, thus permitting them to find a type of support in the vagina of the other specimen, to avoid turbulence caused by water movement during intercourse.
|
|
The sperm exchange takes place simultaneously and this is collected in the so-called seminal receptacle. In the following video of two Trapania hispalensis copulating, one can observe the displacement of the seminal packages that start from the specimen located in the lower part.
The genitalia is located next to the genital atrium and is formed by the structures that we see in the following image. On the left a drawing with the different structures separated and on the right as it is arranged on the animal.
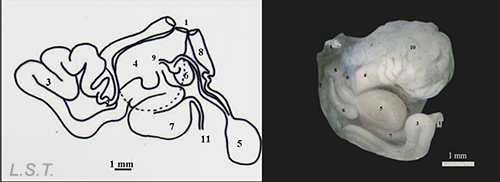
Parts of the genitalia of Dendrodoris grandiflora: 1-Atrio genital, 2-Conducto deferente-, 3- Región prostática del conducto deferente, 4- Glándula femenina, 5- Bolsa copulatriz, 6- Receptáculo , 7- Ampolla, 8- Conducto vaginal, 9 y 10- Conducto uterino , 11- Conducto hermafrodita
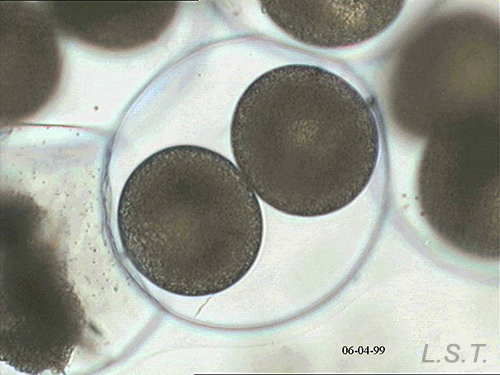
Before oviposition the eggs are fertilized and covered by different protective layers, to defend them from possible predators.
|
|

|
The eggs are deposited directly on the substrate, usually forming spirals and there is no parental care.
|
|
|
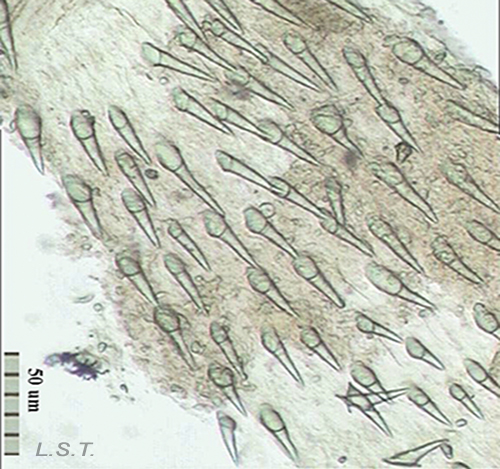
The size of the eggs and the number of eggs per capsule vary from one species to another (see table). The color, size and shape of the eggs laid varies between species.

|

|
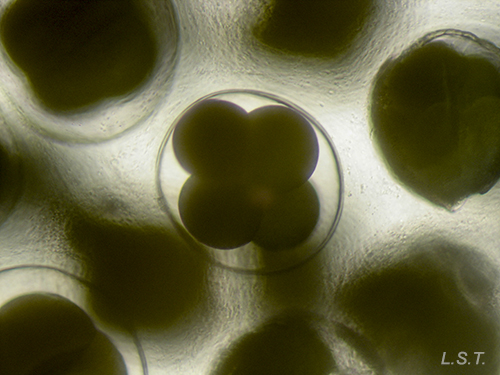
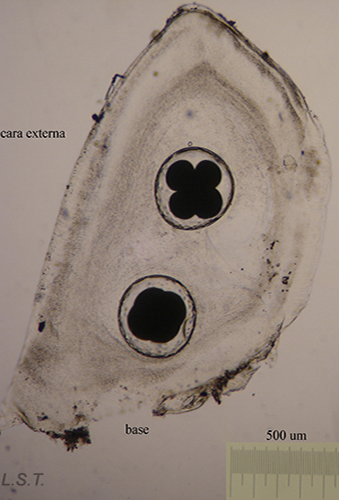
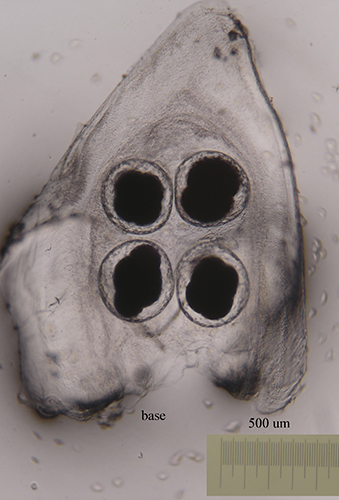
The number of eggs per laying varies and has an influence on the type of larval development. In planctotrophic species (which feed on plankton), for example. Trapania hispalensis, the number of eggs is very high, small and with a shorter time between oviposition and hatching. The veligerous larvae, in this type of development, are almost transparent because they have practically no vitello. Those that present a lecitotrophic type development (they eat yolk), like Dendrodoris limbata, , have a smaller number of eggs and are larger. The larvae are opaque due to the yolk. Finally, in the species with direct development, such as Felimare villafranca, the number of eggs is very small and the velige stage takes place inside the egg.
|
|
|
|
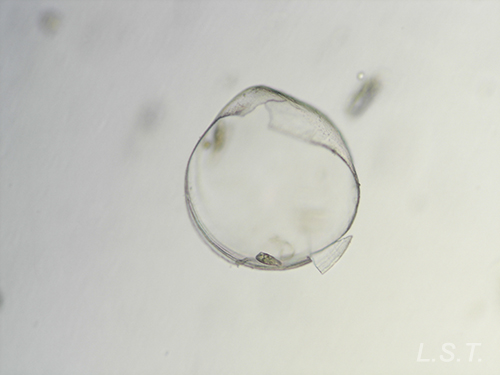
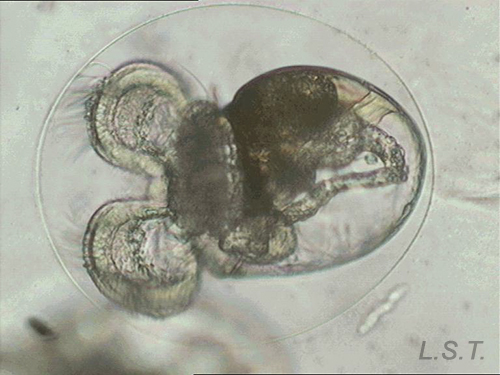
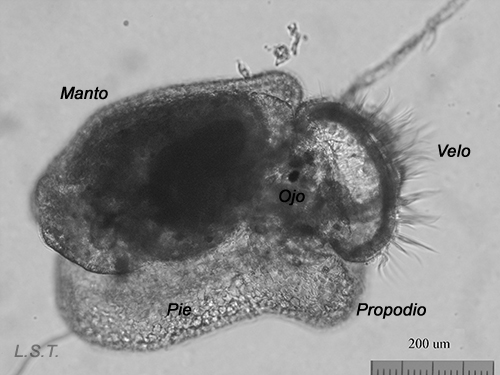
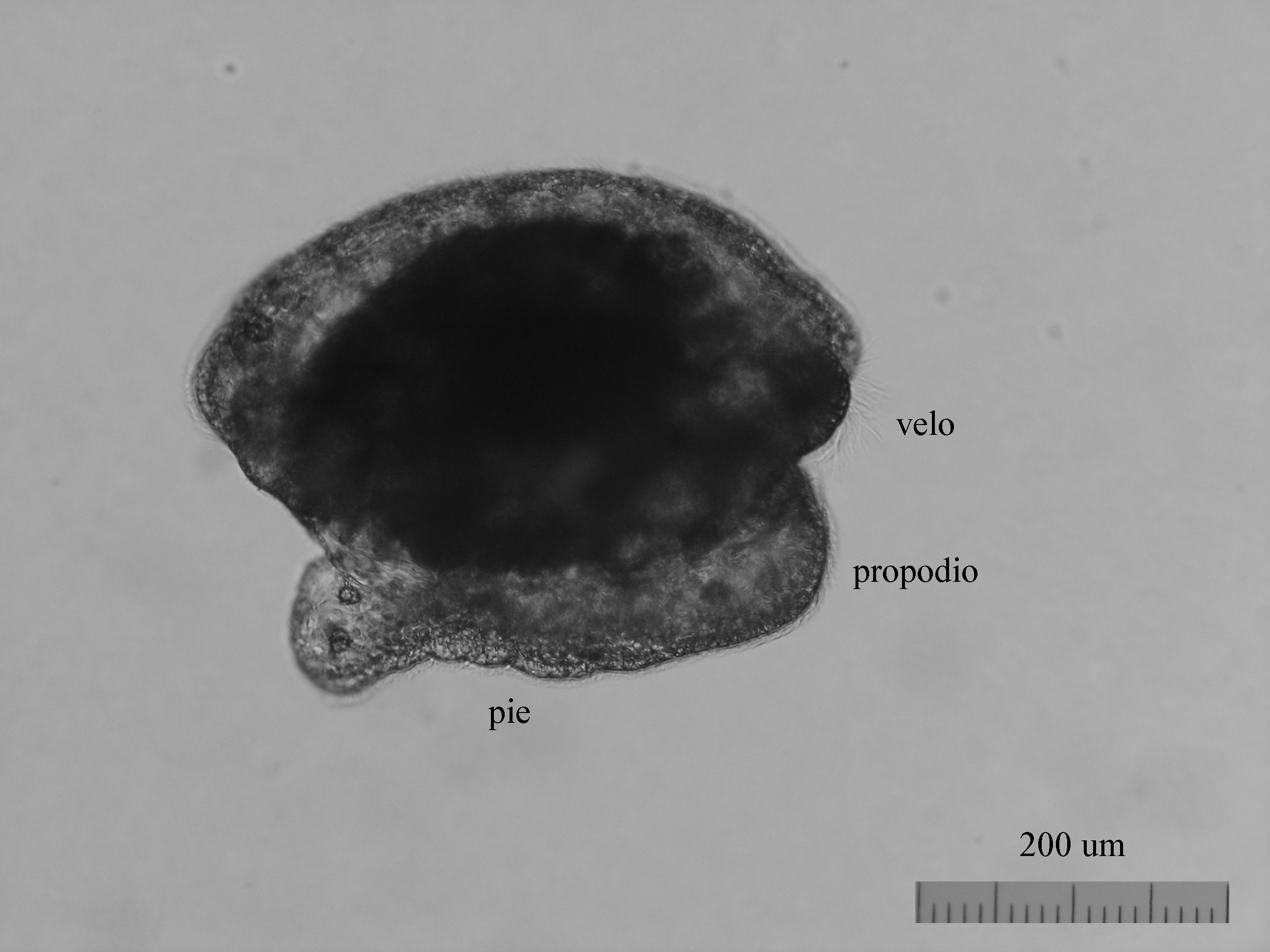
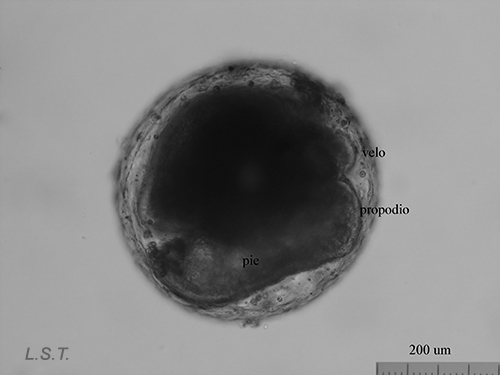
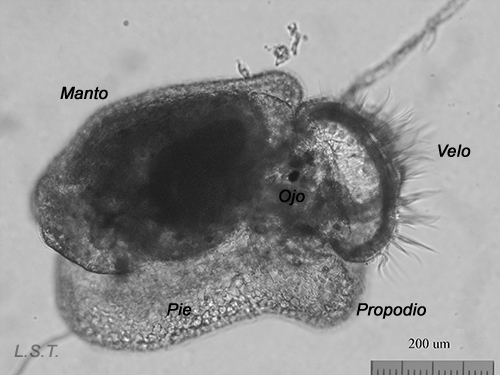
From the egg, after a varied period between some species and others, and which depends on factors such as temperature (at a higher temperature, shorter time), a swimming larva called veligera, planctotrophic or lecitotrophic development, or a small juvenile, direct development ( See table). The lecithotrophic larvae present a smaller veil and lose the shell quickly.
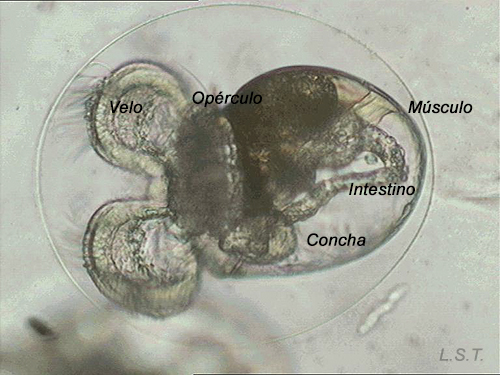
Velíger of F. bilineata (planctotrohic) breaking the egg
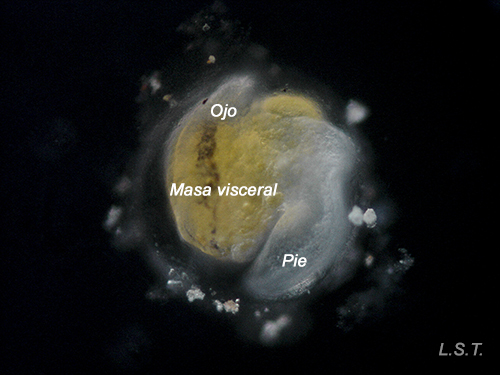
Lecitotrophic veliger of Dendrodoris limbata.
Felimare villafranca (direct development), just before, during and after leaving the egg.
The type of development, logically, has a direct influence on the distribution of the species. Those of planktotrophic development can spend more time in the water column and, therefore, travel long distances pushed by the currents, before the larvae perform the metamorphosis. The lecitotrophic species depend on the yolk for its feeding, which is why this limits its permanency in the plankton, and therefore diminishes its dispersion. The lowest range is that of direct development, when a juvenile egg hatches directly.
 |
 |
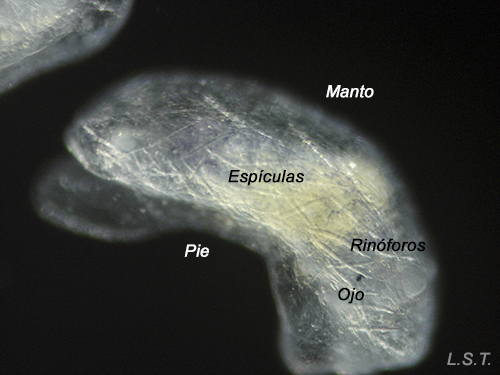
Youths, just a few days old, of Felimare villafranca. One can appreciate the incipient blue coloration and the network of defensive spicules on the mantle.
MORE INFORMATION
TABLE with the characteristics of the laying of some doridáceos of our littoral.
| Species |
Number of eggs per capsule |
Average egg size (µm) |
Embryonic period in days |
Type of development
|
Temperature |
| F. fountandraui | 1 | 110 | 13 | 18º | |
| D. limbata | 1 | 222 | - | - | 18º |
| D. areolata | 1 | 80 | 12 | Planctotrophic | 18º |
| F. picta | 1-2 | 130 | + de 17 | 18º | |
| F. villafranca | 1 | 250 | - | Direct | 18º |
| F. purpurea | 1 | 93 | 13 | Planctotrophic | 18º |
| C. pellucida | 1 | 63 | 8 | Planctotrophic | 20º |
| F. bilineata | 1 | 65 | 11 | Planctotrophic | 20º |
| F. cantabrica | 1 | 85 | - | Planctotrophic | 22º |
| F. tricolor | 1 | 85 | 11 | Planctotrophic | 18º |
| Tayuva lilacina | 1 | 73 | 10 | Planctotrophic | 22º |
| D. rosi | 1 | 80 | 11 | Planctotrophic | 22º |
| A. banyulensis | 1 | - | - | Direct | - |
| J. onubensis | 1-2 | 63 | 9 | Planctotrophic | 22º |
| O. mediterranea | 1 | 77 | - | Planctotrophic | 22º |
| T. maculata | 1 | 100 | 11 | - | 22º |
| T. hispalenses | 1 | 83 | - | Planctotrophic | 22º |
| T. lineata | 1 | - | - | - | 22º |
| C. papillata | 1 | 72 | - | Planctotrophic | 22º |
| Paradoris indecora | 1 | 180 | - | - | 22º |
PHOTOGRAPHS
|
|
|
|
|
|
|
|
|
BIBLIOGRAPHY
Granada coastline
-Sánchez-Tocino, L., Ocaña, A, García-García, F. y Cervera, J.L. 2007. Descripción de las puestas y desarrollo embrionario de algu- nos Doridoidea (Mollusca: Nudibranchia) del Sur de la Península Ibérica. Iberus, 25 (1): 1-20
General
-Ballesteros, M. y Ortea, J.A. 1980. Contribución al conocimiento de los Dendrodorididae (Moluscos: Opistobranquios: Doridáceos) del litoral ibérico. I, Publicaciones Departamento de Zoología, 5: 25-37.
-Bonar, D.B. 1978. Morphogenesis at metamorphosis in opisthobranch molluscs. Pp. 177-196, in F.-S. CHIA&M.E. RICE. Settlement and metamorphosis of marine invertebrate larvae. Elsevier/North-Holland Biomedical Press, New York.
- Coelho, R. & Calado, G., 2010. Spawn and early development of NE Atlantic species of Hypselodoris (Gastropoda: Opisthobranchia). Iberus 28(2): 63–72
.-Domenech, A., Avila, C. y Ballesteros, M. 2002. Spatial and temporal variability of the opisthobranch molluscs of Port LLigat bay, Catalonia, NE Spain. Journal Molluscan Studies, 68: 29-37.
-Fernández- Ovies, C. L. 1979. Puestas, desarrollo y larvas de algunos opistobranquios. Tesis Doctoral, Universidad de Oviedo. 133 pp.
-Fernández- Ovies, C. L. 1981. Contribución a la clasificación morfológica de las puestas de los opistobranquios (Mollusca: Gastropoda). Boletín del Instituto de Estudios Asturianos, Ciencias Naturales, 28: 3-12
-Gantés, H. 1962. Recherches sur quelques larves de Glossodorididae (Mollusque, Opistobranches). Bulletin de la Société des Sciences Naturelles et Physiques du Maroc, 42 : 267-277.
-Miller, M.C. 1961. Annual cycles of some Manx nudibranchs, with a discusión of the problem of migration. Journal of Animal Ecology, 31: 545-569.
-Ros, J. 1981. Desarrollo y estrategias bionómicas en los Opistobranquios. Oecologia aquatica, 5: 147-183.
-Schmekel, L. & Portmann, A. 1982. Opisthobranchia des Mittelmeeres. Springer Verlag, Germany. 410 pp.
-Tchang-si, 1931. Un nouveau cas de condensation embryogényque chez un nudibranche (Doriopsis limbata Cuvier). Comptes Rendues de l´Academie dse Sciences de Paris. 192: 302-304.
-Thompson, T.E. 1967. “Direct development in a nudibranch, Cadlina laevis, with a discussion of developmental processes in Opisthobranchia”. Journal of Marine Biology, Ass U.K., 47: 1-22.
-Thompson, T.E. y Brown, G.H. 1984. Biology of Opisthobranch Molluscs. Vol. 2. Ray Society, London. 229 pp.
-Valdés, A. 1996. Revisión de la superfamilia Porodoridoidea Odhner en Franc 1968 (Mollusca: Nudibranchia) en el Océano Atlántico. Tesis Doctoral. Universidad de Oviedo. Inédita. 179 pp.
-Wilson, N.G. 2002. Egg masses of chromodorid nudibranchs (Mollusca: Gastropoda: Opisthobranchia). Malacologia, 44(2): 289-305.
LUIS SÁNCHEZ TOCINO